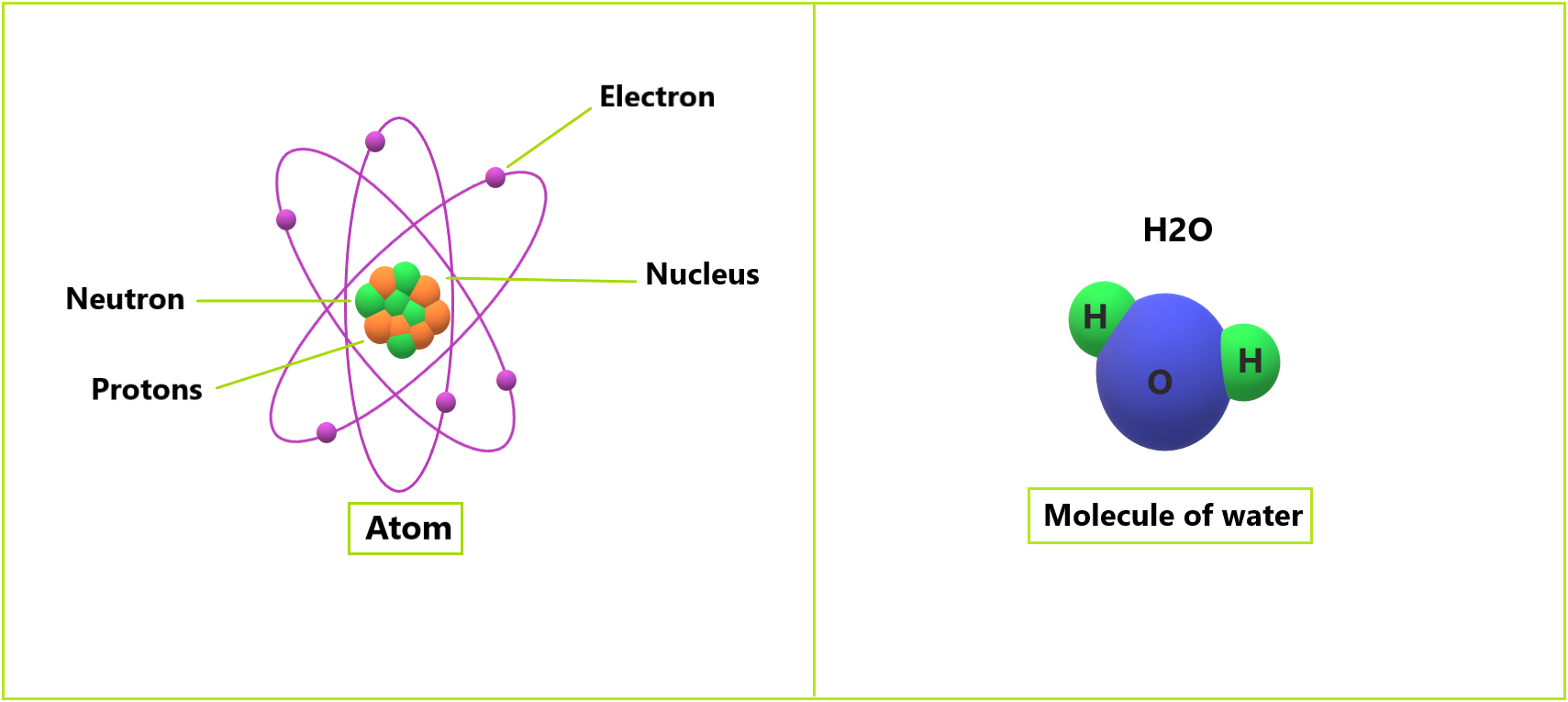
Atomic theory has progressed since ancient Greek times. Scientists have used Greek scholars’ ideas as a starting point for their research. They’ve made numerous discoveries and speculations about the atom. It also comes from the Greek word “atomos,” which means “undivided.” Since then, scientists have discovered that these particles can be broken down further into protons, neutrons, and electrons. Despite this, the term “atom” has endured.
Buy Prime Test Series for all Banking, SSC, Insurance & other exams
Theories of Atomic Structure:
1. Ancient Greeks Belief:
“All matter is made up of atoms, which are small units of matter.” All substance is made up of smaller units called atoms, as hypothesised by Leucippus and Democritus in the fifth century B.C. They further claim that these were solid particles with no interior structure that came in a variety of shapes and sizes. They also say that they were solid particles with no interior structure and that they appeared in a range of shapes and sizes. In addition, they created intangible attributes such as taste and colour.
2. The Dalton Atomic Theory:
In 1808, English scientist John Dalton based his work on the Greek concept of atoms. He proposed that matter is made up of atoms, which he defined as minuscule, indivisible units. He also argued that, while all atoms of one element are identical, those of other elements are completely different.
3. The Theory of J.J. Thomson:
The “plum pudding” idea of the divisible atom was proposed by English scientist Joseph J. Thomson in 1904. After finding electrons in 1897, he did so. In addition, his model suggested that atoms are made up of a large positively charged sphere studded with negatively charged electrons (dubbed “corpuscles”), similar to the fruit in a plum pudding.
He also claimed that the charge of the positive sphere is equivalent to the charge of the electrons’ negative charges. Today, positively charged particles are referred to as protons, while negatively charged particles are referred to as electrons.
4. Rutherford’s Theorem:
In 1911, British physicist Ernest Rutherford suggested a nuclear hypothesis for atoms. A nucleus is a part of an atom. He already identified the central part of the atom’s activity, such as the movement of protons and electrons. He also proposed that the number of protons and electrons in an atom are equal.
5. Bohr’s Theory
Niels Bohr, a Danish physicist, presented a planetary model in 1913, in which electrons rotate around the nucleus in the same way that planets orbit the sun. The electrons have “constant energy” when they are in orbit. This hypothesis refers to these atoms as “stimulated” electrons when they grasp the energy and go into a higher orbit. They leave this energy as electromagnetic radiation when they return to their initial orbit.
The electrons inside atoms are put in discrete orbits called “stationery orbits,” according to the following postulates:
- Quantum numbers can be used to represent the energy levels of these shells.
- Electrons can absorb energy to move to higher energy levels and lose or release energy to go to lower energy levels.
- There will be no energy absorption or emission as long as an electron remains stationary.
- Only in these stationary orbits do electrons spin around the nucleus.
- The stationary orbits’ energy is quantised.
Bohr’s Atomic Theory’s Limitations:
- Only single electron species such as H, He+, Li2+, Be3+, etc. work with Bohr’s atomic structure.
- Each line spectrum was seen to be a composite of a number of smaller distinct lines when the emission spectra of hydrogen was studied using a more accurate spectrometer.
- Bohr’s theory failed to explain both the Stark and Zeeman effects.
6. Quantum Mechanics and Einstein, Heisenberg
- According to previous beliefs, the atom consists of a central and hefty nucleus surrounded by a number of electrons. Previously, electrons and other small particles were thought to be fixed solid “lumps.”
- Modern quantum theory, on the other hand, refers to them as statistical “clouds.” Furthermore, one can precisely measure their speed as well as their location. We can’t, however, accomplish both at the same time.
- According to Heisenberg, no two conjugate physical values can be measured with 100 percent accuracy at the same time. There will always be some measurement error or uncertainty.
Drawback: Bohr accurately measured two such conjugate quantities: position and momentum (theoretically).
- The Stark effect is a phenomenon that occurs when electrons are deflected in the presence of an electric field.
- The Zeeman effect is a phenomenon that occurs when electrons are deflected by a magnetic field.
Matter: Dual Nature
The photoelectric effect reveals that the electrons that were viewed as particles also have a wave nature. With the use of his double-slit experiment, Thomas Young demonstrated this. De-Broglie concluded that because nature is symmetrical, light or any other matter-wave should be as well.
Quantum Mechanics:
The orbital number or shell number of an electron is denoted by the principal quantum number (n).
Azimuthal Quantum Numbers (l): It denotes the electron’s orbital (sub-orbit).
The number of energy levels in each orbit is denoted by the magnetic quantum number. The direction of spin is denoted by the quantum number(s) S = -112 = Anticlockwise and 12 = Clockwise.
Atom Electronic Configuration:
The electrons in the s, p, d, and f must be filled according to the following rule.
- The Aufbau principle states that electrons should be filled in the ascending order of energy of orbitals: The lower energy levels should be filled first, followed by the higher energy levels.
The energy of orbital α(p + l) value it two orbitals have same (n + l) value, E α n
Ascending order of energy 1s, 2s, 2p, 3s, 3p, 4s, 3d, . . .
- Pauli’s exclusion principle: No two electrons can have the same four quantum numbers, and if two electrons must be placed in the same energy state, they must be in opposite spies.
- Hund’s maximum multiplicity rule: Hund’s maximum multiplicity rule states that when filling degenerate (same energy) orbitals, all degenerate orbitals must be singly filled first, followed by pairing.
Find More Miscellaneous News Here



 Scotland Legalises Water Cremation: Firs...
Scotland Legalises Water Cremation: Firs...
 National Safety Day 2026: Why March 4 Ma...
National Safety Day 2026: Why March 4 Ma...
 World Obesity Day 2026: 8 Billion Reason...
World Obesity Day 2026: 8 Billion Reason...








