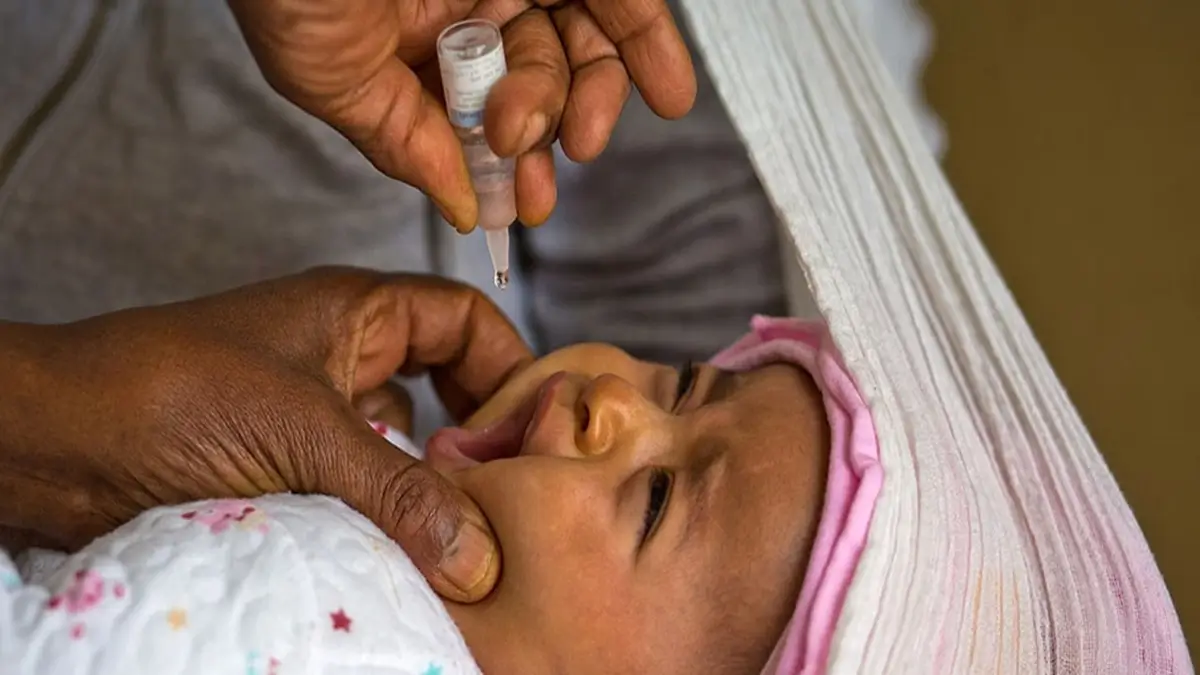
World Health Organization (WHO) has prequalified an additional novel oral polio vaccine type 2 (nOPV2). The move strengthens global capacity to respond to outbreaks caused by circulating vaccine-derived poliovirus type 2 (cVDPV2).
WHO prequalification certifies that the vaccine meets international standards for safety, quality, and effectiveness. This enables UN agencies such as UNICEF to procure and distribute it globally.
What is nOPV2?
The novel oral polio vaccine type 2 (nOPV2) is designed to combat outbreaks caused by vaccine-derived poliovirus type 2, which can emerge in areas with low immunisation coverage.
Key features:
- Genetically more stable than older OPV vaccines
- Lower risk of mutating into virulent strains
- Retains ability to rapidly stop virus transmission
- Shelf life of up to 24 months
- Can be stored under standard vaccine temperatures
The newly prequalified vaccine is produced by Biological E Limited, based in Hyderabad. This expands global production beyond the earlier manufacturer in Indonesia, improving supply resilience.
Global Significance
Polio cases worldwide have declined by more than 99% since the 1980s due to vaccination drives. However, outbreaks of cVDPV2 still occur in under-immunised communities.
The prequalification:
- Strengthens outbreak response capacity
- Diversifies vaccine supply
- Enhances rapid global deployment
- Supports final push toward polio eradication
WHO Director-General Tedros Adhanom Ghebreyesus has reiterated that vaccines remain central to eliminating polio worldwide.
Why It Matters for India
India was certified polio-free in 2014. However, maintaining high immunisation coverage remains critical to prevent re-emergence.
The development is significant because:
- Ensures access to improved, stable vaccine supply
- Strengthens India’s outbreak preparedness
- Enhances India’s role as a global vaccine manufacturing hub
- Supports routine immunisation and mass campaigns
India operates one of the world’s largest immunisation programmes, and the availability of diversified nOPV2 supply adds confidence to sustaining polio-free status.
Key Points for Exams
- WHO prequalified additional nOPV2 vaccine
- Targets circulating vaccine-derived poliovirus type 2 (cVDPV2)
- Manufactured by Biological E Limited (India)
- India declared polio-free in 2014
- Enhances global outbreak response and vaccine supply diversification
The prequalification marks another milestone in the global effort to eradicate polio and reinforces India’s leadership in vaccine production and public health.
Exam-Oriented MCQs
Q1. The World Health Organization (WHO) has prequalified which vaccine to strengthen the global fight against polio?
(a) Inactivated Polio Vaccine (IPV)
(b) Oral Polio Vaccine Type 1 (OPV1)
(c) Novel Oral Polio Vaccine Type 2 (nOPV2)
(d) Bivalent OPV
(e) mRNA Polio Vaccine
Q2. The nOPV2 vaccine is primarily designed to address outbreaks caused by:
(a) Wild poliovirus type 1
(b) Wild poliovirus type 3
(c) Circulating vaccine-derived poliovirus type 2 (cVDPV2)
(d) Measles virus
(e) Rotavirus
Q3. The newly prequalified nOPV2 vaccine is manufactured by which Indian company?
(a) Serum Institute of India
(b) Bharat Biotech
(c) Biological E Limited
(d) Zydus Lifesciences
(e) Panacea Biotec
Q4. India was officially declared polio-free in which year?
(a) 2010
(b) 2012
(c) 2013
(d) 2014
(e) 2015
Q5. WHO prequalification of vaccines primarily ensures:
(a) Low production cost
(b) Global marketing rights
(c) Compliance with safety, quality, and efficacy standards
(d) Patent protection
(e) National licensing only
Q6. One key advantage of nOPV2 over older oral polio vaccines is:
(a) Higher storage temperature requirement
(b) Reduced genetic stability
(c) Lower mutation risk into virulent strains
(d) Intravenous administration
(e) Single-dose lifetime immunity


 Which Banks Rule the World in 2026? Top ...
Which Banks Rule the World in 2026? Top ...
 List Of Outcomes Of Finland President Vi...
List Of Outcomes Of Finland President Vi...
 Deadly Cassava Virus Threatens Africa’s ...
Deadly Cassava Virus Threatens Africa’s ...








