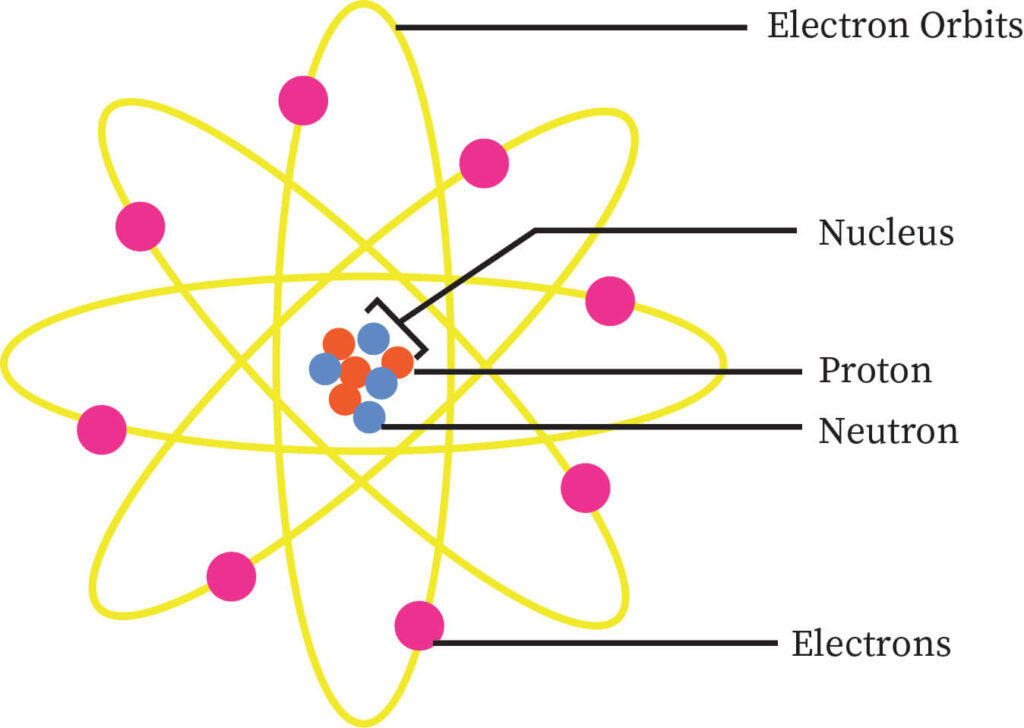
Atomic structure is the structure of an atom that consists of a nucleus (the centre) and protons (positively charged) and neutrons (neutral). The electrons, which are negatively charged particles, circle around the nucleus’s centre.
Atomic Structure (Notes) Theories 2022
Background:
- The origins of atomic structure and quantum mechanics can be traced back to Democritus, the first person to postulate that matter is made up of atoms.
- The study of an atom’s structure provides a wealth of information on chemical processes, bonds, and their physical properties.
- In the 1800s, John Dalton proposed the first scientific theory of atomic structure.
- Other fundamental particles have been discovered as a result of improvements in atomic structure and quantum mechanics. Many more discoveries and innovations have been based on the discovery of subatomic particles.
Atomic structure:
The composition of an element’s nucleus and the arrangement of electrons around it are referred to as its atomic structure. Protons, electrons, and neutrons are the building blocks of matter’s atomic structure.
Nucleus:
- The nucleus of the atom is made up of protons and neutrons, which is surrounded by the atom’s electrons.
Atomic Number:
- The total number of protons in an element’s nucleus is described by its atomic number.
Key Points:
- Protons and electrons are in equal amounts in neutral atoms. Atoms, on the other hand, can receive or lose electrons to strengthen their stability, and the resulting charged entity is known as an ion.
- Because various elements have different numbers of protons and electrons, their atomic structures differ.
- This is why various elements have distinct qualities.
Models at the Atomic Level:
- Many scientists attempted to describe the structure of the atom using atomic models in the 18th and 19th centuries.
- Each of these models had its own set of advantages and disadvantages, and they were all important in the creation of the present atomic model.
- Scientists including John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr made significant contributions to the discipline.
The Atomic Theory of Dalton:
All matter, according to English chemist John Dalton, is made up of indivisible and indestructible atoms. He also claimed that all atoms of a given element were the same, but that the size and mass of atoms of different elements differed.
According to Dalton’s atomic theory, chemical reactions require the rearrangement of atoms to generate products. The atomic structure, according to Dalton’s postulates, was made up of atoms, the smallest particles responsible for chemical reactions.
The following are his theory’s postulates:
- Atoms are the building blocks of all matter.
- Atoms are unbreakable.
- There is just one sort of atom in each element.
- Each atom has a fixed mass that varies depending on the element.
- During a chemical reaction, atoms rearrange themselves.
- Atoms cannot be generated or destroyed, but they can be converted from one state to another.
The Laws of chemical reactions, such as the Law of conservation of mass, the Law of constant properties, the Law of multiple proportions, and the Law of reciprocal proportions, were successfully described by Dalton’s atomic theory.
The Advantages and Disadvantages of Dalton’s Atomic Theory:
- The occurrence of isotopes was not explained by the idea.
- Nothing concerning the atom’s structure was adequately explained.
- Scientists later identified particles inside the atom, proving that atoms are divisible.
- The discovery of subatomic particles inside atoms led to a better understanding of chemical species.
Buy Prime Test Series for all Banking, SSC, Insurance & other exams
Find More Miscellaneous News Here


 Indian Olympic Medal Winners List Till N...
Indian Olympic Medal Winners List Till N...
 Who is the Inventor of the Gramophone?
Who is the Inventor of the Gramophone?
 HS Dhaliwal Appointed New DGP Of Andaman...
HS Dhaliwal Appointed New DGP Of Andaman...
