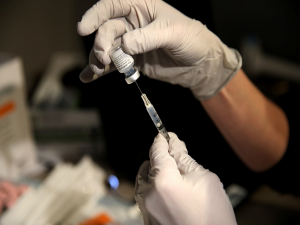
An anti-COVID-19 therapeutic drug developed by DRDO called drug2-deoxy-D-glucose (2-DG) has been given emergency approval by the Drug Controller General of India (DCGI) for Coronavirus patients in the country. The Institute of Nuclear Medicine and Allied Sciences (INMAS), a lab of Defence Research and Development Organisation (DRDO), has developed the drug, in collaboration with Dr Reddy’s Laboratories, Hyderabad.
Buy Prime Test Series for all Banking, SSC, Insurance & other exams
In 2-DG arm, significantly higher proportion of patients improved symptomatically and became free from supplemental oxygen dependence (42% vs 31%) by day-3 in comparison to SOC, indicating an early relief from oxygen therapy/dependence. The drug comes in the form of a powder in a sachet & is supposed to be dissolved in water.
Important takeaways for all competitive exams:
- Chairman DRDO: Dr G Satheesh Reddy.
- DRDO Headquarters: New Delhi.
- DRDO Established: 1958.



 India Joins 100+ Nations in Strong Stand...
India Joins 100+ Nations in Strong Stand...
 Which Musical Instrument is known as the...
Which Musical Instrument is known as the...
 Andhra Pradesh Bags India’s Largest ₹8,1...
Andhra Pradesh Bags India’s Largest ₹8,1...








