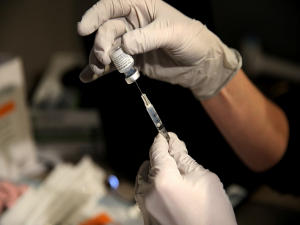
An anti-COVID-19 therapeutic drug developed by DRDO called drug2-deoxy-D-glucose (2-DG) has been given emergency approval by the Drug Controller General of India (DCGI) for Coronavirus patients in the country. The Institute of Nuclear Medicine and Allied Sciences (INMAS), a lab of Defence Research and Development Organisation (DRDO), has developed the drug, in collaboration with Dr Reddy’s Laboratories, Hyderabad.
Buy Prime Test Series for all Banking, SSC, Insurance & other exams
In 2-DG arm, significantly higher proportion of patients improved symptomatically and became free from supplemental oxygen dependence (42% vs 31%) by day-3 in comparison to SOC, indicating an early relief from oxygen therapy/dependence. The drug comes in the form of a powder in a sachet & is supposed to be dissolved in water.
Important takeaways for all competitive exams:
- Chairman DRDO: Dr G Satheesh Reddy.
- DRDO Headquarters: New Delhi.
- DRDO Established: 1958.


 Paris Olympics 2024 Medal Tally, India M...
Paris Olympics 2024 Medal Tally, India M...
 Which District of Madhya Pradesh is Famo...
Which District of Madhya Pradesh is Famo...
 EC Signs Electoral Cooperation Pact with...
EC Signs Electoral Cooperation Pact with...

