Introduction
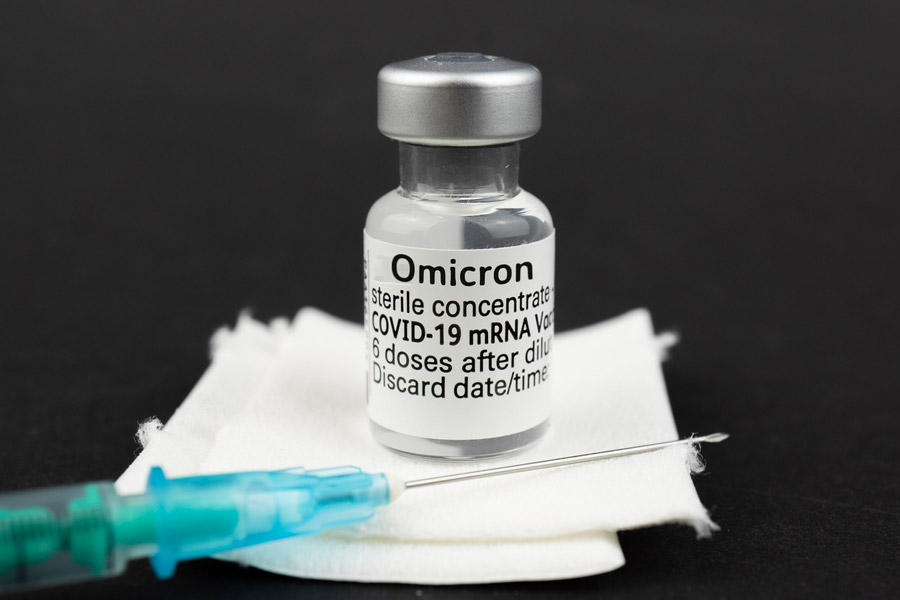
India’s Department of Biotechnology (DBT) has announced the approval of Pune-based Gennova Biopharmaceuticals for emergency use authorization from the Drugs Controller General of India (DCGI) for its mRNA COVID-19 booster vaccine, GEMCOVAC-OM. The vaccine, designed specifically against the Omicron variant of SARS-CoV2, has shown promising results in phase 3 clinical trials, indicating its potential to prevent future waves of the pandemic.
Addressing the Omicron Variant Challenge
With the emergence of the Omicron variant of SARS-CoV2 and the limited efficacy shown by original vaccines against this lineage, a booster dose has become essential. GEMCOVAC-OM is specifically designed to counter the Omicron variant and is expected to address the gap left by existing vaccines.
Launch and Availability
The launch of GEMCOVAC-OM is expected within the next two to three weeks, with New Delhi being the initial location for the vaccine’s introduction. The cost and anticipated demand for the vaccine will be announced during this launch. Currently, 1.2 million booster doses are available at the Central Drugs Laboratory (CDL) in Kasauli, which oversees the national regulations for domestically produced vaccines.
Safety and Administration
GEMCOVAC-OM can be safely administered as a booster dose to individuals aged 18 and older who have already received two doses of either Covaxin or Covishield. The vaccine is stable at 2-8 °C and is in a lyophilized (freeze-dried) form. It is delivered intradermally using the Tropis device developed by PharmaJet, USA.
Indigenous Platform Technology Development
The DBT has played a crucial role in establishing Gennova’s mRNA-based next-generation vaccine manufacturing, from proof of concept to Phase I clinical trials for the prototype mRNA-based vaccine against the Wuhan strain. The project received further support through the Mission COVID Suraksha initiative for clinical development and scale-up of the prototype vaccine. The platform technology developed through this initiative has now been utilized to create an Omicron-specific booster vaccine for COVID-19.
The Future of mRNA Vaccines
Gennova Biopharmaceuticals is the third company globally, after Moderna and Pfizer, and the first in India, to develop an mRNA COVID-19 booster vaccine against the highly transmissible Omicron variant. Dr Singh emphasized that the mRNA platform provides a rapid response capability for developing vaccines to counter any future variants of concern. Gennova’s mRNA vaccine technology was developed in collaboration with the Department of Biotechnology, Government of India.
About Department of Biotechnology
- It is an Indian government department, under the Ministry of Science and Technology responsible for administrating development and commercialisation in the field of modern biology and biotechnology in India.
- Its headquarters are in New Delhi.
- Jitendra Singh is the Minister of Science and Technology.
Important Takeaways for Competitive examination
- Dr Sanjay Singh is the CEO of Pune-based Gennova Biopharmaceuticals Ltd.
- Rajeev Raghuvanshi Drug Controller General of India (DCGI).


 Indian Olympic Medal Winners List Till N...
Indian Olympic Medal Winners List Till N...
 Who is the Inventor of the Gramophone?
Who is the Inventor of the Gramophone?
 HS Dhaliwal Appointed New DGP Of Andaman...
HS Dhaliwal Appointed New DGP Of Andaman...
