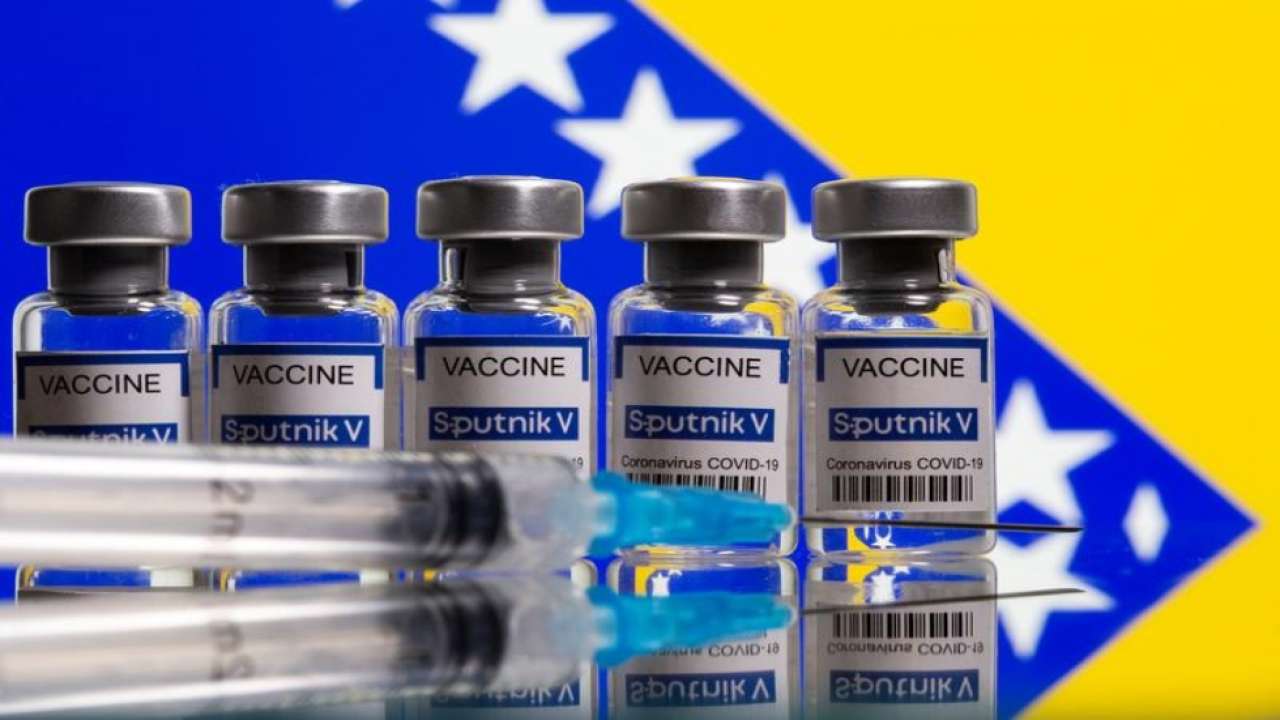
The Central drug regulator, DCGA has approved emergency use authorisation of the Russian Vaccine, Sputnik V. It has now become the third vaccine to get emergency use authorisation from the drug regulator after Covishield and Covaxin. The Vaccine was developed by Gamaleya National Research Institute of Epidemiology and Microbiology last year in Russia.
About Sputnik V clinical trial:
- The clinical trials of SPUTNIK V in India is being done by Dr Reddy’s Lab. The Hyderabad based, multinational Indian pharma company, Dr Reddy’s Lab has also inked an agreement with the Russian Direct Investment Fund, RDIF for the supply of the Russian vaccine in India.
- The Subject Expert Committee of the Indian drug regulator had yesterday recommended SPUTNIK V for emergency use authorisation.
- The vaccine has displayed robust results in its clinical trials in the country.
- RDIF has claimed effectiveness of 91.6 per cent for the Sputnik V vaccine. Dr Reddy’s had applied for emergency use of Sputnik-V in February this year.
Important takeaways for all competitive exams:
- Russia President: Vladimir Putin.
- Russia Capital: Moscow.
- Russia Currency: Russian Ruble.


 Scotland Legalises Water Cremation: Firs...
Scotland Legalises Water Cremation: Firs...
 National Safety Day 2026: Why March 4 Ma...
National Safety Day 2026: Why March 4 Ma...
 World Obesity Day 2026: 8 Billion Reason...
World Obesity Day 2026: 8 Billion Reason...








