Batteries are crucial devices used to store and release electrical energy for various applications, from powering mobile electronics to electric vehicles. The invention of the battery revolutionized technology, providing a reliable source of energy. This article explores the history, development, and future of batteries, including their advantages and disadvantages.
Who Invented the Battery and When?
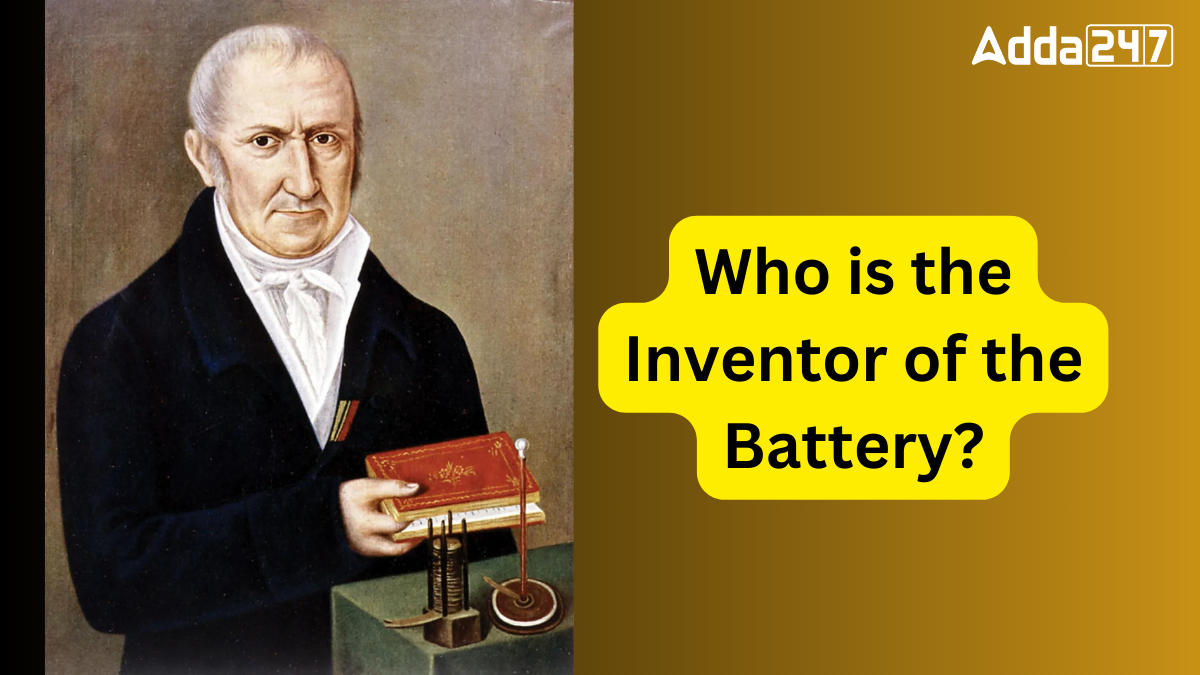
The first true battery, known as the “Voltaic Pile,” was invented by Italian physicist Alessandro Volta in 1800. The Voltaic Pile produced a continuous electrical current by alternating layers of copper and zinc discs, separated by saltwater-soaked cardboard. This groundbreaking invention laid the foundation for modern electrical power sources.
How was the Battery Invented?
Alessandro Volta’s innovation in 1800 involved stacking alternating copper and zinc discs separated by saltwater-soaked cardboard. This arrangement created a steady flow of electricity, marking the first practical battery capable of consistent electrical output. Over time, battery technology evolved, leading to the advanced batteries used in today’s electronics.
Volta’s Experiments with the Electric Battery in1796
In 1796, Volta’s experiments with his Voltaic Pile represented a major breakthrough in electricity generation. Before this, scientists could only generate static electricity through friction. The Voltaic Pile enabled a continuous flow of electricity, significantly advancing scientific research and leading to subsequent innovations such as the telegraph and electric motor.
Early Batteries
Early forms of batteries date back to ancient times. The Baghdad Battery, from the 3rd century BC, consisted of a clay jar with a copper cylinder and iron rod, possibly used for electrotherapy. Similarly, the Vitruvian Battery of the 1st century BC, attributed to Vitruvius, was a bronze device likely used for similar purposes. However, it was Alessandro Volta’s 1800 invention of the Voltaic Pile that marked the first practical and reliable battery.
The Invention of the Rechargeable Battery
The concept of rechargeable batteries emerged with French engineer Georges Leclanché’s zinc-carbon cell in 1866, which could be recharged by passing current through it. This was followed by the development of lead-acid batteries in the late 19th and early 20th centuries. The first commercially successful rechargeable battery, nickel-cadmium (NiCad), appeared in the late 1940s, followed by nickel-metal hydride (NiMH) batteries in the 1980s. These advancements have enabled widespread use in portable devices and electric vehicles.
How do Batteries Work?
Batteries convert chemical energy into electrical energy through electrochemical reactions. They consist of two electrodes (anode and cathode) and an electrolyte. When connected to a circuit, electrons flow from the negative to the positive electrode, creating an electric current. The chemical reactions within the battery enable this flow and can be reversed during recharging.
The Future of Battery Technology
The future of batteries promises significant advancements. Lithium-ion batteries are expected to become more efficient with longer life and faster charging. Emerging technologies, such as solid-state batteries, offer improved safety, higher energy density, and faster charging. These innovations will likely enhance electric vehicles and renewable energy storage systems.
Advantages of Batteries
- Portability: Batteries provide a convenient power source for various devices without needing an external power supply.
- Energy Storage: They store energy for use when there is no immediate power source.
- High Energy Density: Batteries can deliver a substantial amount of energy in a compact form.
- Cost-Effective: They offer a more economical solution for powering small devices compared to alternatives like generators.
Disadvantages of Batteries
- Limited Lifespan: Batteries need to be replaced periodically.
- Environmental Impact: Battery production can be environmentally harmful, with some materials being toxic.
- Discharging Speed: Batteries may discharge slower than other power sources, limiting use for power-hungry devices.
- Risk of Explosion: Damaged batteries can pose safety hazards, including the risk of fire or explosion.
- Cost of Replacement: Ongoing replacement costs can be significant over time.



 Which River is known as the Moon River o...
Which River is known as the Moon River o...
 Which is the Highest Mountain of Austral...
Which is the Highest Mountain of Austral...
 Which Country is known as the Land of Mo...
Which Country is known as the Land of Mo...








