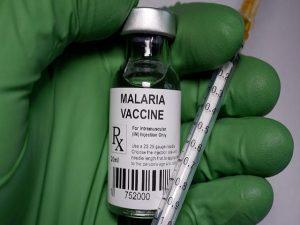
World Health Organization (WHO) is recommending widespread use of the RTS,S/AS01 (RTS,S) malaria vaccine among children in sub-Saharan Africa and in other regions with moderate to high P. falciparum malaria transmission. The recommendation is based on results from an ongoing pilot programme in Ghana, Kenya & Malawi that has reached more than 800 000 children since 2019.
Buy Prime Test Series for all Banking, SSC, Insurance & other exams
The vaccine is developed by British drugmaker GlaxoSmithKline (GSK). Many vaccines exist against viruses and bacteria but this was the first time that the WHO recommended broad use of a vaccine against a human parasite. The vaccine acts against Plasmodium falciparum, one of five parasite species and the most deadly. The symptoms of malaria are fever, headaches, and muscle pain, then cycles of chills, fever, and sweating.
Important takeaways for all competitive exams:
- President of the World Health Organisation: Tedros Adhanom.
- Headquarters of WHO: Geneva, Switzerland.
- WHO founded: 7 April 1948.


 Indian Olympic Medal Winners List Till N...
Indian Olympic Medal Winners List Till N...
 Who is the Inventor of the Gramophone?
Who is the Inventor of the Gramophone?
 HS Dhaliwal Appointed New DGP Of Andaman...
HS Dhaliwal Appointed New DGP Of Andaman...
