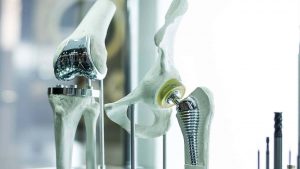
The Government of India has decided to consider all medical devices including implants and contraceptives as “Drugs”. The notification regarding the re-categorization was issued by the Ministry of Health and Family Welfare. With the implementation, all the medical devices will come under the purview of the Central Drugs and Standard Control Organization (CDSCO). The re-categorization will be implemented from 1st April 2020 in the phase manner. The re-categorization will enable the drugs regulator “CDSCO” to tighten the regulation to improve the safety and quality.
The medical devices that will be re-categorized as per the notification issued by the Ministry of Health and Family Welfare, includes the devices that are used for life support, diagnosis, treatment or alleviation of any disease or disability. It also includes the devices that are used to disinfect other medical devices. This notification will effectively cover all medical devices that are sold in the market.
Ministry of Health and Family Welfare will also release another notification stating a transition period for various categories of medical devices.
Present rules for the classification of medical devices in India:
In India, the medical devices are classified under four categories:
- Class A
- Class B
- Class C
- Class D
Examples of medical devices as per their category:
- Class A and B medical devices are low-risk ones like surgical dressing, alcohol swabs, thermometers, blood pressure monitoring devices.
- Class C and D are high-risk devices like implants, hemodialysis catheters, angiographic guide wire and heart valve.
According to the present medical rules, CDSCO issues licenses to the private notified bodies for the inspection of low risk-devices. But, in case of high risk devices, CDSCO is responsible for the issuing of licenses as well as for the inspection of the high risk devices.
Most Important takeaways for upcoming competitive exams:
- Union Minister of Ministry of Health and Family Welfare: Harsh Vardhan.


 Which District of Uttar Pradesh is known...
Which District of Uttar Pradesh is known...
 Which Mountain is known as the Stone Sen...
Which Mountain is known as the Stone Sen...
 Brazil Creates History! Lucas Pinheiro B...
Brazil Creates History! Lucas Pinheiro B...








