Context:
Donanemab, a new drug for Alzheimer disease has shown positive results in the clinical trial according to the study published in the New England Journal of Medicine.
More about the research:
Donanemab drug has been tested on 257 participants with early-stage Alzheimer, who were randomly assigned to receive either donanemab or placebo by intravenous infusion every four weeks for 76 weeks.
The researchers measured a change in a cognitive and functional score called the Integrated Alzheimer’s Disease Rating Scale (IADRS) which ranges from 0-144. The researchers also measured the change in amyloid-beta levels in the brain using emission tomography (PET) scans.
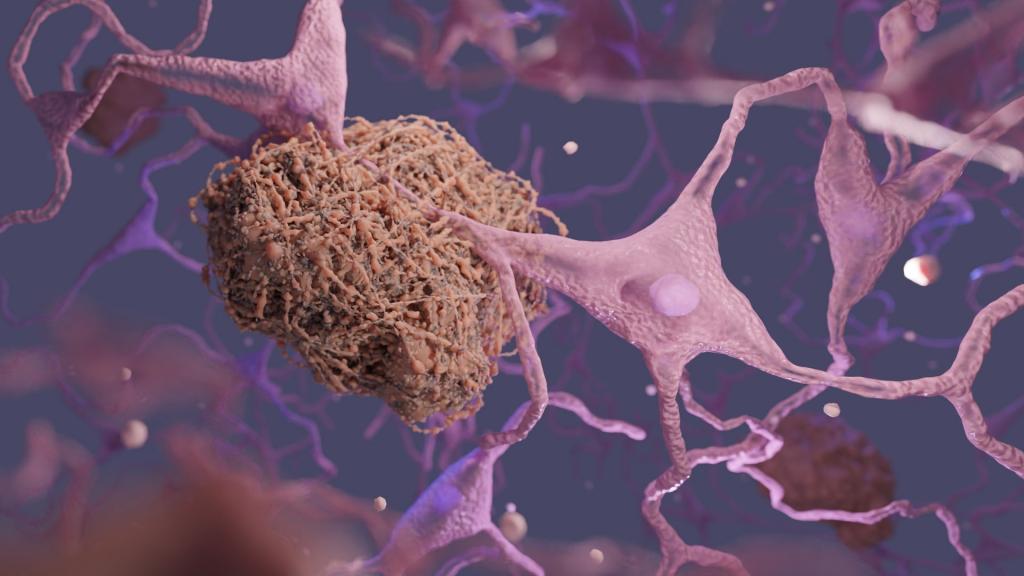
What is Donanemab?
Donanemab is an antibody therapy from the US pharmaceutical giant Eli Lily which targets the abnormal clumps of protein called amyloid-beta that can build up in brain. Donanemab is given as an intravenous infusion once every four weeks. Patients need to have regular brain scans to monitor drug’s side effects, including brain swelling and bleeding.
Effectiveness of the drug:
- According to the study, Donanemab is not a cure. Patients of Alzheimer did not improve but they deteriorated more slowly than a control group that received a placebo.
- After decades of failed trials and billions of dollars invested in research it is proved that drugs can alter the course of the disease is regarded as a significant triumph.
- On an average, the drug slowed the progression of the disease by 20-30% amounting to about 4-7 months over the course of 18-month trial.
Risk with Donanemab:
There are significant side-effects of the donanemab. In the trial of drug, nearly a quarter of patients treated experienced with brain swelling or bleeding, compared with only 2% in the control group, though serious problems were rare. Four people died while taking part in the trial, three in the donanemab group and one in the control group.



 Legendary Bengali Author Shankar Passes ...
Legendary Bengali Author Shankar Passes ...
 List of Dadasaheb Phalke Award Winners f...
List of Dadasaheb Phalke Award Winners f...
 Which Dance Form is known as the Ballad ...
Which Dance Form is known as the Ballad ...








